Microbes live all around us, and in us, and are central to health and wellbeing. A lot of what microbes do they do in dense groups where social interaction is rife. Sometimes they help each other by secreting signals and growth-promoting enzymes. Sometimes they harm each other with powerful toxins and molecular spearguns. Why then do some microbes cooperate with one another while others engage in competition and warfare? Cooperation within a microbial community can bring with it both virulent disease and antibiotic resistance. The outcome of competition is key to the composition of microbial communities and whether a pathogen can invade. Understanding microbial interactions, therefore, is important for both biology and medicine.
When will cooperation evolve within a species of microbe?
We study microbes by applying the theory that was first developed to predict the balance between cooperation and conflict in humans and other animals, including game theory, kin selection theory and multilevel selection theory. This work has helped to identify a number of key processes that affect the balance between cooperation and competition in microbes.
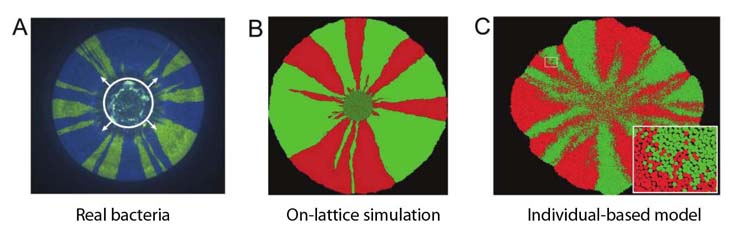
1. Emergent genetic structure. Microbes undergo genetic bottlenecks as they divide on a surface, which leads to regions of genetically-identical cells that share a common evolutionary interest. We have shown how this process can lead to cooperation using an individual-based model and followed the effects of bottlenecks on genetic diversity in colonies of real bacteria, showing that spatial structure is important for traits such as cooperative mechanisms of antibiotic resistance.
Xavier, J.B., and Foster, K.R. 2007 Cooperation and conflict in microbial biofilms. Proceedings of the National Academy of Sciences, 104: 876-881; Foster, K. R. and Xavier, J. B. 2007 Cooperation: Bridging ecology and sociobiology. Current Biology, 17: R319-R321; Nadell CD, Xavier J, Levin SA, & Foster, KR 2008 The evolution of quorum sensing in bacterial biofilms. PLoS Biology, 6: e14, Nadell CD, Xavier J, & Foster, KR 2009 The sociobiology of biofilms. FEMS microbiology reviews, 33: 206-224; Nadell CD, Foster KR, Xavier J. 2010 Emergence of spatial structure in cell groups and the evolution of cooperation. PLoS Computational Biology, 6: e1000716; Korolev KS, Xavier JB, Nelson DR, Foster KR 2011. A quantitative test of population genetics using spatio-genetic patterns in bacterial colonies. American Naturalist, 178: 538-552.; Kim W, Racimo F, Schluter J, Levy SB, Foster KR. 2014. Importance of Positioning for Microbial Evolution. Proceedings of the National Academy of Sciences, 111, E1639–E1647; Schluter S, Schoech A, Foster KR, Mitri S. 2016. The evolution of quorum sensing as a mechanism to infer kinship. PLoS Computational Biology, 12: e1004848; Nadell CD, Drescher K, Foster KR. 2016 Spatial structure, cooperation, and competition in biofilms, Nature Reviews Microbiology, 14: 589-600; Frost I, Smith W, Mitri S, San Millan A, Davit Y, Osborne JM, Pitt-Francis JM, Maclean C, Foster KR. 2018. Cooperation, competition and antibiotic resistance in bacterial colonies. ISME journal, 12: 1582–1593.
.jpg) 2. Incomplete cell separation. Another way to form groups of genetically identical cells is to not divide properly. We have shown that the budding yeast Saccharomyces cerevisiae does this and that this can help cells to use cooperative enzymes. Is this the reason why multicellularity first evolved?
2. Incomplete cell separation. Another way to form groups of genetically identical cells is to not divide properly. We have shown that the budding yeast Saccharomyces cerevisiae does this and that this can help cells to use cooperative enzymes. Is this the reason why multicellularity first evolved?
Koschwanez J, Foster KR, Murray AJ. 2011. Sucrose utilization in budding yeast as a model for the origin of undifferentiated multicellularity. Plos Biology, 9(8): e1001122; Koschwanez J, Foster KR, Murray AJ. 2013. Improved use of a public good selects for the evolution of undifferentiated multicellularity. eLife, 2:e00367.
3. Genetic recognition. Cells can directly recognise other cooperating cells and preferentially interact with them, which prevents non-cooperators from benefiting from the investments of cooperators. We have shown that cells of the budding yeast Saccharomyces cerevisiae are capable of this impressive form of discrimination by virtue of a single gene FLO1. This produces a sticky protein that allows cells to find other cells making the protein by the simple virtue that they are more likely to stick to each other.
Smukalla S, Caldara M, Pochet N, Beauvais A, Guadagnini S, Yan C, Vinces MD, Jansen A, Christine Prevost M, Latge J, Fink GR, Foster KR, Verstrepen KJ 2008. FLO1 is a variable green beard gene that drives biofilm-like cooperation in budding yeast. Cell, 135: 727-737
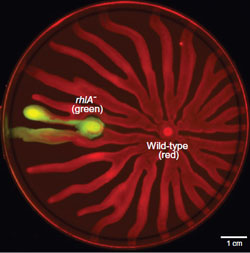 4. Metabolic prudence. if cells only produce cooperative traits when it is cheap to do so, then they can be prosocial at little or no cost to themselves. We have found that Pseudomonas aeruginosa does just this, secreting carbon-rich rhamnolipids that help other cells when there is excess carbon in the environment.
4. Metabolic prudence. if cells only produce cooperative traits when it is cheap to do so, then they can be prosocial at little or no cost to themselves. We have found that Pseudomonas aeruginosa does just this, secreting carbon-rich rhamnolipids that help other cells when there is excess carbon in the environment.
Xavier J, Kim W, Foster KR 2011 A molecular mechanism that stabilizes cooperative secretions in Pseudomonas aeruginosa. Molecular Microbiology 79, 166-179
5. Pleiotropic constraints. If cooperative traits are genetically linked to traits that provide a benefit to the individual cells, then this will constrain mutations that produce non-cooperative phenotypes because the mutation will also remove the individual benefit. We have shown that such genes exist in the slime mould Dictyostelium discoideum.
Foster KR., Shaulsky G, Strassmann, J. E., Queller, D. C., Thompson, C. R. L. 2004. Pleiotropy as a mechanism to stabilise cooperation. Nature 431: 693-696; Foster, K.R., Parkinson, K. and Thompson, C. R. L. 2007 What can microbial genetics teach sociobiology? Trends in Genetics, 23:73-80; Foster KR. 2011 The sociobiology of molecular systems. Nature Reviews Genetics, 12: 193-203. Bentley MA, Yates CA, Hein J, Preston GM, Foster KR 2022 Pleiotropic constraints promote the evolution of cooperation in cellular groups. PLoS Biology, 20(6): e3001626
When will cooperation evolve between different species of microbes?
We believe that cooperation between different microbial species is relatively rare. Our models suggest that cooperation among different species is difficult to maintain as it requires that the cells of one species can both help another, and that this help can be returned. Moreover, we have assessed the propensity for cooperation among different microbial species and it appears to be rare. However, competition between strains is common and a driver of many processes in microbial communities, both evolutionary and ecological.
Foster, K.R., Wenseleers, T. 2006. A general model for the evolution of mutualisms Journal of Evolutionary Biology, 19: 1283-1293; Mitri S, Xavier J, Foster KR 2011 Social evolution in multispecies biofilms Proceedings of the National Academy of Sciences, 108: 10839-46; Foster KR, Bell T. 2012. Competition, not cooperation, dominates interactions among culturable microbial species. Current biology 22, 1845–1850; Cornforth DM, Foster KR 2013 Competition sensing: the social side of bacterial stress responses. Nature Reviews Microbiology, 4, 285-93; Oliveira NM, Niehus R, Foster KR. 2014 The evolutionary limits to cooperation in microbial communities. Proceedings of the National Academy of Sciences, 111: 17941-17946; Oliveira NM, Martinez-Garcia E, Xavier J, Durham WM, Kolter R, Kim W, and Foster KR. 2015. Biofilm formation as a response to ecological competition. PLoS Biology, 13: e1002191; Coyte K Tabuteaue, H, Gaffney EA, Foster KR, Durham WH 2017. Microbial competition in porous environments can select against rapid biofilm growth. Proceedings of the National Academy of Sciences, 114: E161–E170; Rakoff-Nahoum S, Foster KR, Comstock L. 2016. The evolution of cooperation within the gut microbiota. Nature, 533: 255–259. Palmer JD and Foster KR 2022. Bacterial species rarely work together. Science, 376: 581-582
Bacterial Warfare
Bacteria stab, poison and kill their competitors with an amazing diversity of molecular weapons. While bacterial weapons are well-studied mechanistically, less attention has been paid to how bacterial weapons are actually used and what determines whether bacteria succeed or fail in a contest. To highlight this, we wrote a review of bacterial warfare that identifies the questions for the field (Granato et a. 2019 Current Biology). We have also built a body of research around these questions that combines theory and experiments. We have studied when bacterial weapons are useful (Mavridou et al. 2018 Current Biology; Smith et al. 2020 PLoS Biology) and how they are actually used in combat (Smith et al. 2020 Nature Communications; Granato et al. 2020 Current Biology; Niehus et al. 2021 eLife; Oliveira et al 2022 Nature Communications), which has shown the importance of competition sensing i.e. bacteria will often detect threats and fight back (Cornforth and Foster 2013 Nature Review Microbiology; Lories et al. 2020 PLoS Biology). One of our recent studies won the Gilbert S. Omenn Prize for the best paper of the year in evolution and medicine (Palmer and Foster 2022 PNAS).
The ecology and evolution of the human microbiome
Microbial communities are critical for health, agriculture, industry and the environment. A key current goal, therefore, is to both understand and engineer these communities, including the human microbiome. However, this is a challenging prospect because most microbial communities contain large numbers of evolving and interacting species, which makes them complex systems with many moving parts. As a result, progress in microbiome research has arguably been slow with many studies showing links to health but a notable lack of overarching principles. Our goal is to identify general principles for understanding and engineering the microbiome. To do this, we combine ecological and evolutionary theory with wide range of methods including individual-based modelling, molecular microbiology, bioinformatics, singe-cell timelapse microscopy, large-scale ecological experiments with gut bacteria, and germ-free mouse work. We seek to understand what it takes for a given bacterial strain to succeed in diverse communities and what determines the properties of communities as a whole, including the ability to resist pathogens.
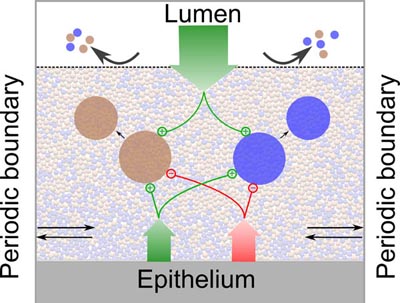
Schluter J, Foster KR. 2012. The evolution of mutualism in gut microbiota via host epithelial selection. PLoS Biology, 10(11): e1001424; Schluter J, Nadell CD, Bassler BL and Foster KR. 2015 Adhesion as a weapon in microbial competition. ISME journal, 9, 139-149; Coyte KZ, Schluter J, Foster KR. 2015. The ecology of the microbiome: networks, competition, and stability. Science, 350: 663-666; McLoughlin K, Schluter K, Rakoff-Nahoum S, Smith A, Foster KR. 2016. Host selection of microbiota via differential adhesion. Cell Host and Microbe, 19: 550–559. Rakoff-Nahoum S, Foster KR, Comstock L. 2016. The evolution of cooperation within the gut microbiota. Nature, 533: 255–259; Foster KR, Schluter J, Coyte KZ and Rakoff-Nahoum S. 2017. The evolution of the host microbiome as an ecosystem on a leash. Nature, 548: 43-51; Johnson K, Foster KR. 2018. Why does the microbiome affect behaviour? Nature Reviews Microbiology, 16:647–655. Coyte KZ, Rao C, Rakoff-Nahoum S, Foster KR. 2021 Ecological rules for the assembly of microbiome communities. PLoS Biology 9:e3001116. Sharp C, Foster KR. 2022. Host control and the evolution of cooperation in host microbiomes. Nature Communications,13: 3567